Mass-energy and Nuclear Binding Energy
Mass-energy and Nuclear Binding Energy: Overview
This topic covers concepts such as Nuclear Mass, Equivalence of Mass and Energy, Equivalent Energy of 1 U Mass, Mass Defect, Binding Energy, Binding Energy per Nucleon, Dependence of Nuclear Stability on Binding Energy per Nucleon, etc.
Important Questions on Mass-energy and Nuclear Binding Energy
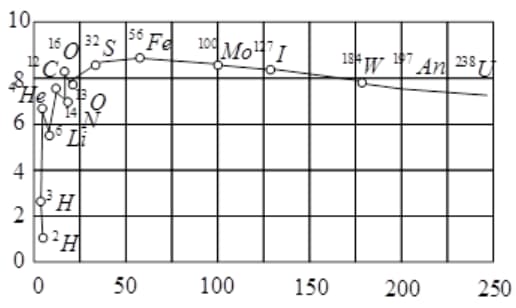
The plot of the binding energy per nucleon versus the mass number A for a large number of nuclei, is shown in Fig. In which range the binding energy per nucleon is constant?
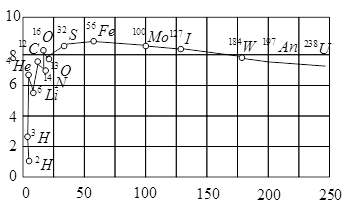
The plot of the binding energy per nucleon versus the mass number A for a large number of nuclei, is shown in Fig. In which range the binding energy per nucleon is constant?
Calculate the energy released in MeV in the following nuclear reaction :<
[Mass of = 238.05079 u]
Mass of = 234.043630 u
Mass of = 4.002600 u
1 u = 931.5 MeV
Calculate the binding energy per nucleon of nucleus.
Given:
Mass of ,
Mass of proton ,
and
In the nuclear process, stands for _______
Binding energy of a nucleus is,
The binding energy per nucleon is maximum in the case of,
If an -nucleus is completely converted into energy, the energy produced will be around
Define:
Binding energy of electron
Calculate binding energy for nucleus. [Given, atomic mass of , mass of neutron , mass of proton and ]
Explain mass effect and binding energy? Explain the main conclusions which can be drawn from the binding energy per nucleon versus mass number diagram.
Which of the following nucleus having highest binding energy per nucleon:
The average binding energy per nucleon(in ) of having mass is . Write the value of , where denotes the greatest integer function.
(Given , and )
Let be the mass of proton, the mass of a neutron, the mass of a nucleus, and the mass of a nucleus. Then,
Energy in electron volt corresponding to Compton wavelength is
The mass defect in a particular nuclear reaction is The amount of energy liberated in kilowatt hour is (velocity of light )
The binding energy per nucleon is maximum in the case of
If binding energy per nucleon of is , is and is , then what is value of the reaction
A nucleus has mass represented by . If and denote the mass of proton and neutron respectively and the binding energy (in MeV) then,
